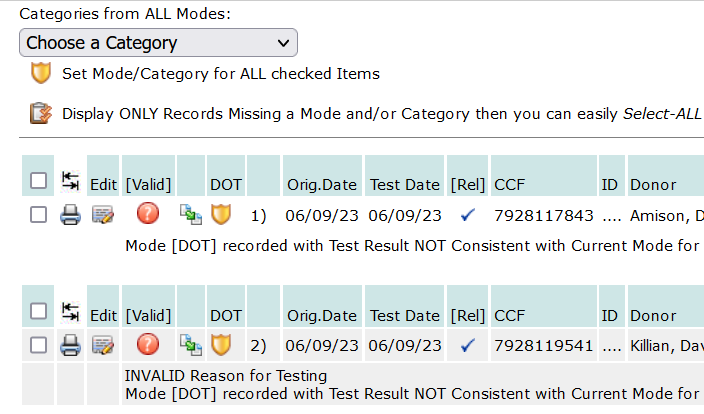
Setting Up an Employee for Random Periodic Drug and Alcohol Testing After a Positive Test and Issue a document with the dates to the Employer:
When an employee tests positive for drugs and/or alcohol in a random drug test, the employee may be required to participate in random periodic testing to protect their employment, ensure compliance, safety, and rehabilitation. This process involves setting up a testing schedule that covers weekly, or monthly testing over a specified period; for example, a year. Scheduled dates are randomly generated based on the Periodic Testing Profile established for the employee as outlined below.
Developing a Testing Schedule: Frequency & TimeFrame:
- Frequency: The number of tests required within a TimeFrame.
- TimeFrame: For example:
- 7 = Weekly
- 30 = Monthly
- 1/7 = once a week
- 2/14= twice every 14 days.
- 1/30= once a month
Setting up the Periodic Profile for an employee:
Open the personnel profile editor for the employee.
Open the section: Periodic Testing: By Color or No. Days/Period & Test Protocols
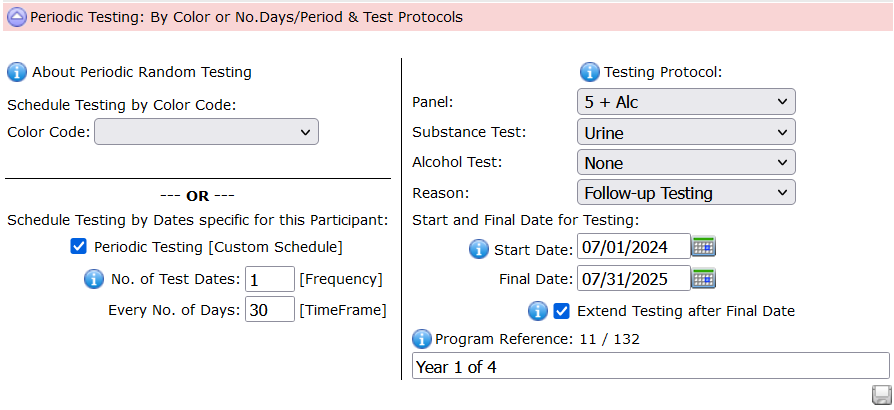
To establish a tailored set of dates for the employee, check the box:
Periodic Testing [Custom Schedule]. Color Testing is outlined in another article.
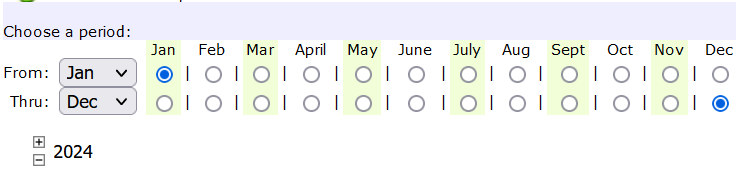
The example above establishes testing once a month between July 1, 2024 through July 31, 2025.
The Testing Protocol outlines the testing required.
Click the info icons ![]() to learn more about each component. Be sure to save the record when you’re done.
to learn more about each component. Be sure to save the record when you’re done.
When the record is saved, click the tools symbol ![]() to open data management options for the employee.
to open data management options for the employee.
From the options choose:![]()
When the page opens, follow the instructions to create random dates.
Issue a document (PDF) with the dates and testing requirements to the company supervisor:
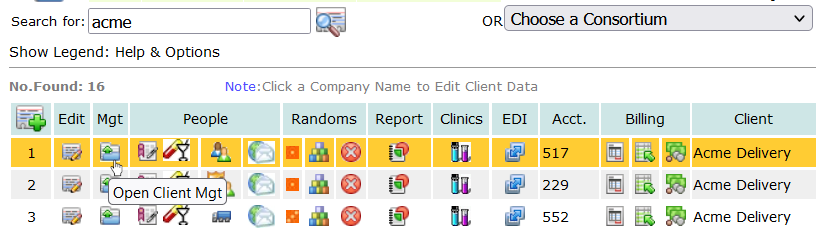
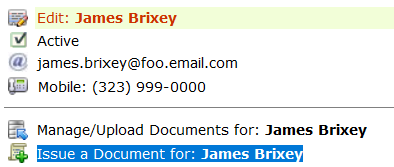
Once you’ve created the random dates for testing, you can issue a document to the employee’s supervisor with an outline of their requirements for testing and the dates for the tests. To issue the document, first locate the data resource options for the employee from the main menu: Personnel.
From the Personnel page, search for the employee, and from the search you’ll see the click the tool symbol ![]() that appears on the line for each person returned by the search query.
that appears on the line for each person returned by the search query.
As shown below, from the Tools/Resource page click the option:
Issue a Document for …
Choose the Document Template SAP / EAP (this is a sample document template that was provided with your subscription to DTN and can be found under Other_Data > Section 6.b General Documents. Using these templates you can create documents that automatically fill-in the company name for the account, contacts, employee name & ID, etc. for any content you might need. The SAP/EAP template is specific to this example. The issue document module recognizes keywords {{keyword}} and replaces the occurrence of {{keyword}} with its respective data element. For example, where {{employee}} appears in template, the module replaces it with the first and last name of the employee from their data record. If you review the SAP/EAP template, you will see the {{keywords}} used for periodic testing and the dates generated.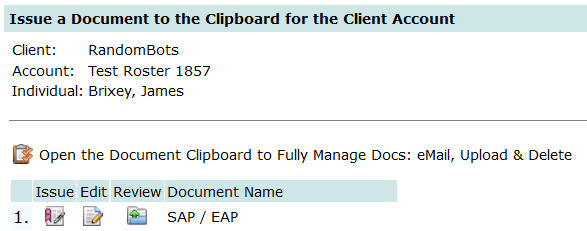
Click the issue symbol ![]() to issue the document.
to issue the document.
When you issue the document, it will get posted to the clients Document Clipboard and emailed to the company contact.