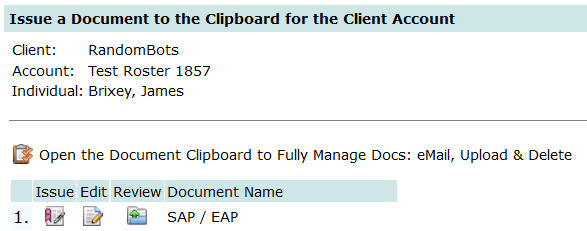
What documents to maintain . . .
The content below was copied from the DOT website on 10/11/2024.
To obtain a recent copy in pdf format:
https://www.transportation.gov/sites/dot.gov/files/docs/ODAPC Recordkeeping Requirements.pdf
Click HERE for the complete document: 49 CFR Part 40: https://www.ecfr.gov/current/title-49/subtitle-A/part-40 [CFR: Code of Federal Regulations].
U.S. Department of Transportation
Office of the Secretary
Office of Drug & Alcohol Policy & Compliance
Employer Record Keeping Requirements
For Drug & Alcohol Testing Information
Requirement: Employers covered under DOT drug & alcohol testing regulations must maintain records that document their testing program consistent with 49 CFR Part 40 and other industry specific regulations.
Click HERE for the complete document: 49 CFR Part 40 document: https://www.ecfr.gov/current/title-49/subtitle-A/part-40 [CFR: Code of Federal Regulations].
Industry specific regulations requiring record keeping are listed in the following:
Industry |
Regulation |
|---|---|
| FAA – Airline | 14 CFR part 120, Subpart E, section 120.11114 CFR part 120, Subpart F, section 120.219 |
| FMCSA – Motor Carrier | 49 CFR part 382.401 |
| FRA – Railroad | 49 CFR part 219.901 and 219.903 |
| FTA – Transit | 49 CFR part 655.71 |
| PHMSA – Pipelines | 49 CFR part 199.227 |
| USCG – Maritime | 46 CFR Part 16.260 |
Note: Employers are required to thoroughly document their program, decision- making and compliance with respective DOT regulations. The drug & alcohol testing documents listed below will only make up a part of the over-all records you will need to keep.
While actually reading the regulations are the best practice, we hope this fact sheet provides a good starting point in answering your questions on record keeping.
What do I keep: Employers are required to keep the following documents:
- Test results.Testing process administration.
- Return-to-duty process administration.
- Employee training.
- Supervisor training.
You may also be required to submit an annual report to a DOT agency regarding testing activity and results. For more information on Management Information Systems (MIS) Data reports, please visit: www.dot.gov/ost/dapc/mis.html.
How do I keep them: All DOT drug & alcohol test records must be kept in a secure location with controlled access. Records should be in locked file cabinets. If records are kept electronically, they should be password protected.
Best Practice: Some employers have found separating their drug & alcohol records from personnel records or medical records limits the access of employees to the drug & alcohol records.
May a consortium or Third Party Administrator keep the records: Yes, you may arrange to have a consortium or third party administrator keep some or all of your records. You do not have to maintain a duplicate set of records, but ultimately, it is the employer’s responsibility to ensure procedures are in place that guarantee accurate and current records are saved according to DOT regulations.
After a request has been made by an authorized representative of the appropriate DOT agency, records need to be made available for inspection at the employer’s principal place of business. Please refer the DOT agency regulation to determine the time frame records need to be made available.
How long must I keep records: Depends on the type of transportation industry in which you are operating. Most DOT agencies have similar requirements, but please see the charts below for a break-down of agency-specific requirements:
FMCSA Requirements |
|
|---|---|
| 1 Year: |
|
| 2 Years: |
|
| 3 Years: |
|
| 5 Years: |
|
Indefinite period: Education and Training records, plus two years after
ceasing to perform functions.
FTA Requirements |
|
|---|---|
| 1 Year: |
|
| 2 Years: |
|
| 3 Years: |
|
| 5 Years: |
|
FAA Requirements |
|
|---|---|
| 1 Year: |
|
| 2 Years: |
|
| 3 Years: |
|
| 5 Years: |
|
PHMSA Requirements |
|
|---|---|
| 1 Year: |
|
| 2 Years: |
|
| 3 Years: |
|
| 5 Years: |
|
FRA Requirements |
|
|---|---|
| 2 Years: |
|
| 3 Years: |
|
| 5 Years: |
|
USCG Requirements |
|
|---|---|
| 1 Year: |
|
| 2 Years: |
|
| 3 Years: |
|
| 5 Years: |
|
Note: The prudent employer establishes a record retention policy that addresses
both the DOT agency minimum requirement and other business information
needs, e.g. statute of limitations for accidents, etc.
Do I have to keep paper files: Yes. Employers may also keep electronic records for
their own purposes, but DOT requires that paper records be kept. Not only does this help answer questions that arise regarding specific documents, such as the federal custody and control form, but the practice facilitates work by inspectors, who have found many companies reluctant to allow them access to their computer systems.
May employees get drug & alcohol records from their current or previous
DOT covered employer: Yes. Upon request, employees are entitled to all records
about their drug & alcohol tests. An employer must provide records promptly. While
some employers require employees to sign a release before releasing records, release of the records cannot be contingent upon receiving any kind of payment from the employee. Employers are required to provide test results and return-to-duty testing records.
May employees get records from the Medical Review Officer (MRO): Yes.
Upon request, the MRO must provide all records that are available related to the
employee within ten working days. In most instances, the MRO requires a release signed by the employee, and the MRO may charge no more than the cost of preparing,
reproducing and shipping the records. Also, the release of the records cannot be
contingent upon receiving any kind of payment from the employee.
As an employer what records do I release to other employers: You must
provide all the information in your possession concerning employee’s DOT drug &
alcohol tests that occurred in the two years (or three years for FMCSA covered
employees) preceding the request.* This includes information you received from a
former employer.
Note: If you provide information about an employee’s DOT drug & alcohol tests
obtained from a former employer dating back more than two (or three) years, this is not a violation of DOT regulations or DOT agency rules.* This does not impact the Pilot Record Improvement Act’s 5-year requirement for pilots applying to work for FAA covered employers. (See 49 USC 44703(h)).
For more information: Visit our website at http://www.dot.gov/ost/dapc or e-mail us (DOT) at ODAPC@dot.gov or call 202.366.DRUG (3784).

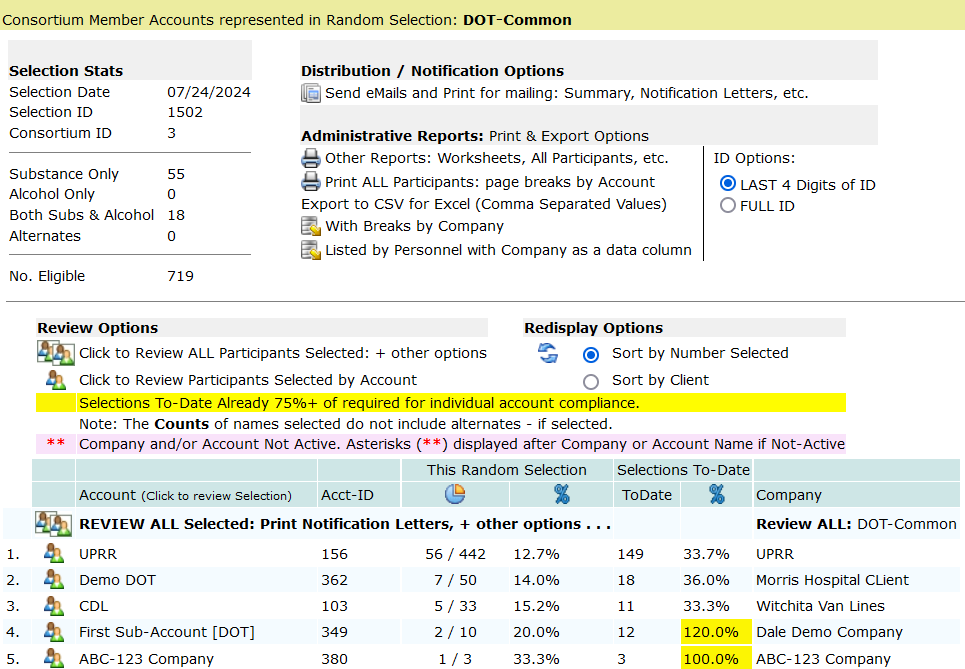



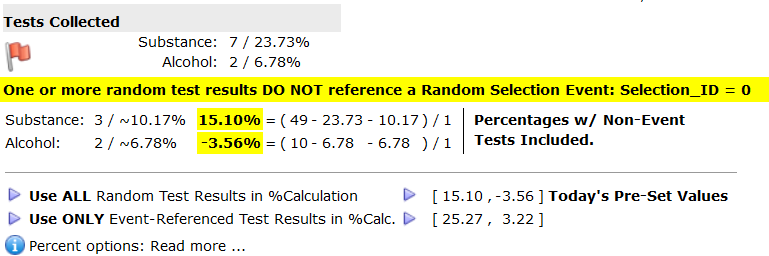
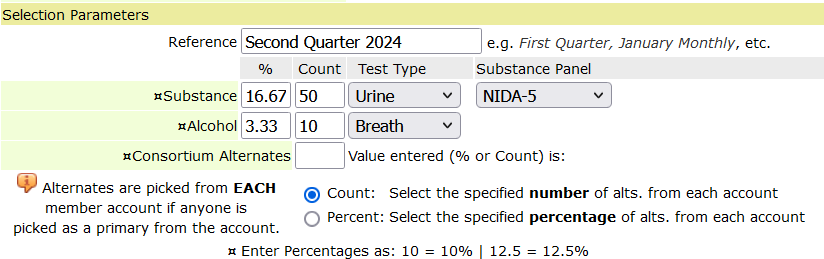
 and click the first symbol on the right
and click the first symbol on the right